Julia’s research programme is not funded by BRUK.
To recap from my last blog, I and my team at Anglia Ruskin University have been collaborating with Queen Mary University (London) and a biotech company called Emulate to develop a new form of IBD treatment consisting of hydrogels primed with healthy gut cells that potentially can serve as a safe, effective and inexpensive therapy in severe cases of Crohn’s disease and ulcerative colitis.
The aim of our treatment, which is in the early stages of development, is to be able to reduce markedly an inflamed gut mucosa that is typical in Crohn’s and ulcerative colitis patients, and to seal a ‘leaky gut’ so that harmful viruses and bacteria cannot enter the bloodstream and intensify the inflammatory reaction.
As IBD patients know all too well, these diseases are chronic and progressive conditions that require lifelong management. While progress has been made understanding some of the causes of IBDs, we need much more research to understand the very complex interplay between genetics, gut microbiota, and environment in an IBD that currently makes it very hard to find a cure.
We are, however, very pleased with our progress so far. Initially, we fabricated temperature-sensitive hydrogel/cell systems in my laboratory. The hydrogels were designed to be liquid at room temperature and become gel-like at 37°C (body temperature). We then performed a wide range of experiments to characterise the systems and see whether they have a toxic effect on the gut cells. Following this, we evaluated the developed systems using Emulate’ s gut-on-a-chip model (Figure 1) utilising the lab facilities at QMUL. The aim of the chip technology is to mimic as closely as possible the human gut, and therefore predict with greater certainty whether new treatments have the potential to work in the human body.
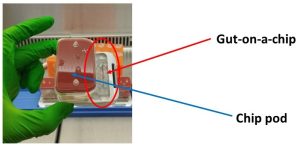
Figure 1
Figure 1: Gut-on-a-chip connected to a pod containing media necessary to maintain the cell growth in the chip.
In our study were able to successfully artificially induce inflammation in the gut-on-a-chips to mimic an active IBD and then we treated the chips with the developed hydrogel/cell systems for 24 hours. For control purposes, at the same time we ran the same experiment with non-treated chips.
Our findings suggest that the new treatment had a restorative and possibly a protective effect on the villi structures in the gut chip. Intestinal villi are cells that line the entire length of our small intestine (Figure 2). Their job is to absorb nutrients from the food we eat and then shuttle them into our bloodstream where they are required. The microscopy results clearly demonstrated the presence of fully differentiated villi structures in the chips that were treated with the hydrogel/cell systems. These villi structures were absent in the not-treated chips.
Figure 2: Small intestine cross- section and diagram of intestinal villi (www.freepik.com).
Currently, we are now getting ready to run a larger study. In this new study we plan to carry out additional preclinical research (i.e. not involving human participants) that optimises our protocols and hopefully further validates our findings. If all goes well, we hope to report our next set of findings in the summer of 2024.
At this preclinical drug development stage, we want to collect information on drug efficacy and safety and how the body interacts with the administered drug. Only once we have reviewed the results from the new study will we be able to determine whether we can move towards testing the drug on people.
The goal of the new treatment is to selectively deliver healthy gut cells to damaged colons. This means that it must be administered rectally to the patient in the form of an enema for example, which could be conducted in a medical setting or even at home.
For some patients, there will be issues with rectally administered treatments, but these have a number of advantages such as reaching immediately the site of need and working faster and with fewer side effects than drugs taken orally. However, we acknowledge if patients feel discomfort after administration this might reduce compliance.
One thing to note, this treatment does not rely the organ-on-a-chip technology. This technology is a kind of proxy for a human-like model and enables us to test and predict whether and how a human body reacts to the proposed treatment and whether it is effective. If the treatment reaches the clinical stage, it can be introduced without the chips.
We strongly believe that our novel treatment approach will have a positive impact on the patients’ quality of life and will ease the often considerable emotional burden of living with an IBD.
By restoring injured gut tissue and preventing a gut wall from leaking, we expect to be able to reduce many IBD symptoms such as pain in the stomach area, rectal bleeding, the number of flares, bloody diarrhoea, the need for hospital admissions, and potential surgery. This, in turn, will lead to increased physical activity, promote well-being, and considerably improve patients’ social lives.
If everything goes smoothly, the treatment could be available in 10 to 12 years, which is the average timespan for taking a drug all the way through from initial studies to licensed treatments. But we are also hopeful that the organ-on-a-chip technology used in this study to better predict the human response might shorten the timeline for bringing a new drug to market. We shall see.
