As many of you reading this know well, inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis are chronic conditions that involve inflammation of the gut. Over time, the gut wall in IBD patients becomes ‘leaky’ and harmful bacteria can enter the blood circulation triggering an immune response and intensifying gut inflammation.
Research in 2022 found that inflammatory bowel disease now affects more than 1 in 123 people which is almost 500 000 people in the UK suffering from these conditions and a big rise on the previous estimate of 300 000 people living with an IBD.
Currently, inflammatory bowel diseases are not medically curable. There are a sizeable number of different types of drug therapies available for doctors to prescribe help to reduce symptoms and avoid disease complications. However, these drug therapies work throughout the body and in some patients can cause severe side effects.
Ultimately, approximately 20% of ulcerative colitis and up to 70% of Crohn’s disease patients will require colon surgery at some point during their disease, according to the World Journal of Gastroenterology. These numbers highlight the urgent need for new treatment options that have the potential to repair the inflamed gut rather than minimising the symptoms and inflammation taking place in the gut.
Which brings me to the study I am running at my research lab at Anglia Ruskin University which I am working on in collaboration with Queen Mary University, London and a biotech company called Emulate. Together we are collaborating on a new form of IBD treatment developing hydrogels that contain healthy gut cells that potentially can serve as a safe, effective and inexpensive therapy in severe cases of inflammatory bowel disease.
Hydrogels are three-dimensional networks of cross-linked natural or synthetic compounds that can swell in body fluids while maintaining their structure. They can be loaded with different types of drugs and deliver them to the sites in the body where they are needed, such as an inflamed gut.
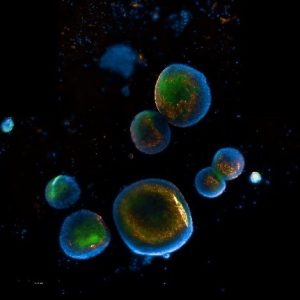
We are developing hydrogels to carry healthy gut cells rather than drugs and deliver them directly to the gut. The source of the healthy cells are human intestinal organoids (see image), which are tiny masses of tissues (also called ‘mini organs’) made from human cells and grown in laboratories. These healthy gut cells are being designed to stick to the gut wall and release the cells at the site of inflammation. Research showed that cells derived from organoids could replace the old cells in the body and allow it to work more efficiently in reducing inflammation and promoting tissue repair.
Before we employ hydrogels in IBD treatments, we need to understand how effective they might be compared to existing therapies. My study will employ the latest technology called ‘the organ-on-a-chip technology’. The ‘gut-on-a-chip’ consists of human gut tissue grown on tiny silicon chips that can mimic the human gut. We believe that they will better predict what happens in the human gut compared to standard laboratory models.
By artificially inducing inflammation in the gut-on-a-chip and then treating the chip with the developed hydrogel-cell systems we will be able to test whether they can heal the leaky gut.
What’s really exciting is that this new local and targeted treatment approach has the potential to locally restore the inflamed gut and initiate wound repair. This could significantly improve the treatment of inflammatory bowel disease and enhance patients’ quality of life.
